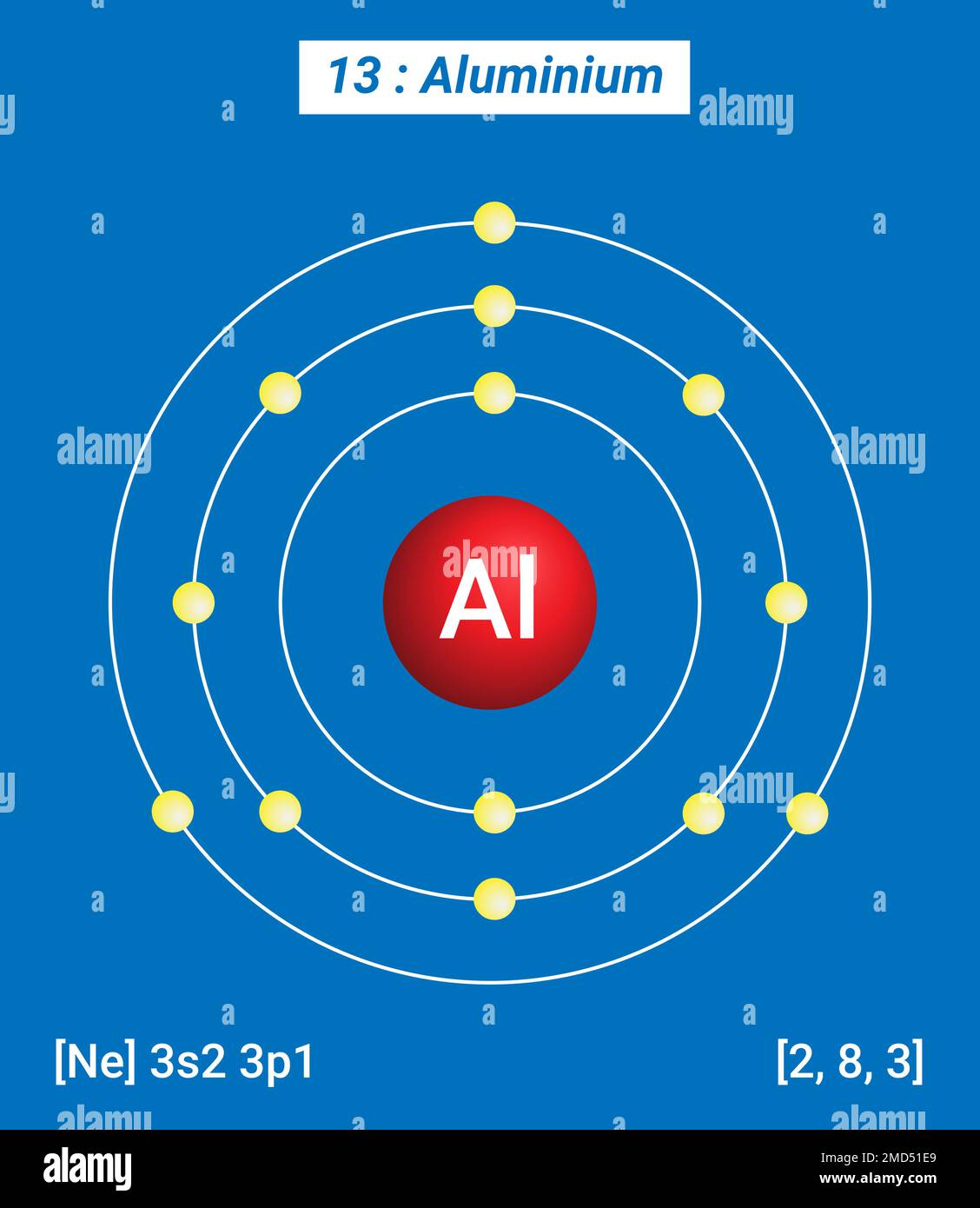
Aluminum Atom Have Electrons . The proton number is also 13. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. To find the electron configuration of an atom, you first need to know the number of electrons. It has an atomic weight of. Groupa vertical column in the periodic table. Members of a group typically have similar properties and electron. Aluminium has 13 electrons and 14 neutrons. electron configuration of aluminum. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a.
from www.alamy.com
an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. Members of a group typically have similar properties and electron. The proton number is also 13. Aluminium has 13 electrons and 14 neutrons. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. electron configuration of aluminum. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: Groupa vertical column in the periodic table. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. To find the electron configuration of an atom, you first need to know the number of electrons.
Atom model aluminium Stock Vector Images Alamy
Aluminum Atom Have Electrons Groupa vertical column in the periodic table. aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: The proton number is also 13. Groupa vertical column in the periodic table. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. Aluminium has 13 electrons and 14 neutrons. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Members of a group typically have similar properties and electron. To find the electron configuration of an atom, you first need to know the number of electrons. electron configuration of aluminum. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. It has an atomic weight of.
From www.alamy.com
Atom model aluminium Stock Vector Images Alamy Aluminum Atom Have Electrons It has an atomic weight of. Members of a group typically have similar properties and electron. The proton number is also 13. Aluminium has 13 electrons and 14 neutrons. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: To find the electron configuration of an atom, you first need to know. Aluminum Atom Have Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Have Electrons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Members of a group typically have similar properties and electron. Aluminium has 13 electrons and 14 neutrons. The proton number is also 13. aluminum is the 13th element in the periodic table and has a symbol of al and. Aluminum Atom Have Electrons.
From quizizz.com
Review Lesson Ionic Bonds and Naming Science Quizizz Aluminum Atom Have Electrons To find the electron configuration of an atom, you first need to know the number of electrons. Groupa vertical column in the periodic table. The proton number is also 13. It has an atomic weight of. aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Aluminium has 13. Aluminum Atom Have Electrons.
From www.dreamstime.com
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminum Atom Have Electrons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. electron configuration of aluminum. The proton number is also 13. It has an atomic weight of. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. Members of. Aluminum Atom Have Electrons.
From ar.inspiredpencil.com
Aluminium Atomic Structure Aluminum Atom Have Electrons electron configuration of aluminum. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. The proton number is also 13. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. [ne]3s 2 3p 1. Aluminum Atom Have Electrons.
From valenceelectrons.com
What is the Electron Configuration for Aluminum and Al3+? Aluminum Atom Have Electrons electron configuration of aluminum. It has an atomic weight of. Groupa vertical column in the periodic table. Members of a group typically have similar properties and electron. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: all atoms have the same number of electrons as protons, so the positive. Aluminum Atom Have Electrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Aluminum Atom Have Electrons The proton number is also 13. electron configuration of aluminum. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. It has an atomic weight of. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Aluminium has. Aluminum Atom Have Electrons.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminum Atom Have Electrons [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. aluminum is the 13th element in the periodic table and has a symbol of al and. Aluminum Atom Have Electrons.
From www.dreamstime.com
Atom of Aluminum with Detailed Core and 13 Electrons on White with Aluminum Atom Have Electrons Aluminium has 13 electrons and 14 neutrons. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. To find the electron configuration of an atom, you first need to. Aluminum Atom Have Electrons.
From brainly.in
Atomic structure of aluminum Brainly.in Aluminum Atom Have Electrons electron configuration of aluminum. aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. Members of a group typically have similar properties and electron. Aluminium has 13 electrons and 14 neutrons. Groupa vertical column in the periodic table. an aluminium atom has 13 electrons, arranged in an. Aluminum Atom Have Electrons.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Aluminum Atom Have Electrons Groupa vertical column in the periodic table. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. To find the electron configuration of an atom, you first need to know the number. Aluminum Atom Have Electrons.
From www.theinternet.io
Ask AI Show me the diagram of an aluminium atom Aluminum Atom Have Electrons To find the electron configuration of an atom, you first need to know the number of electrons. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery:. Aluminum Atom Have Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons And Electrons Elcho Table Aluminum Atom Have Electrons It has an atomic weight of. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. electron configuration of aluminum. The proton number is also 13. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery:. Aluminum Atom Have Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Aluminum (Al)? Aluminum Atom Have Electrons an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [ 17 ] with three electrons beyond. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2. Aluminum Atom Have Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Aluminum Atom Have Electrons aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. electron configuration of aluminum. an aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. The proton number is also 13. It has an atomic weight of. Groupa. Aluminum Atom Have Electrons.
From www.coursehero.com
[Solved] Draw the full electron orbital diagram for a neutral aluminum Aluminum Atom Have Electrons To find the electron configuration of an atom, you first need to know the number of electrons. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: It has an atomic weight of. an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [. Aluminum Atom Have Electrons.
From mavink.com
Aluminum Shell Diagram Aluminum Atom Have Electrons Aluminium has 13 electrons and 14 neutrons. [ne]3s 2 3p 1 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 1 (full) discovery: Members of a group typically have similar properties and electron. electron configuration of aluminum. It has an atomic weight of. Groupa vertical column in the periodic table. an aluminium atom has 13 electrons,. Aluminum Atom Have Electrons.
From newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminum Atom Have Electrons Groupa vertical column in the periodic table. aluminum is the 13th element in the periodic table and has a symbol of al and atomic number of 13. To find the electron configuration of an atom, you first need to know the number of electrons. all atoms have the same number of electrons as protons, so the positive and. Aluminum Atom Have Electrons.